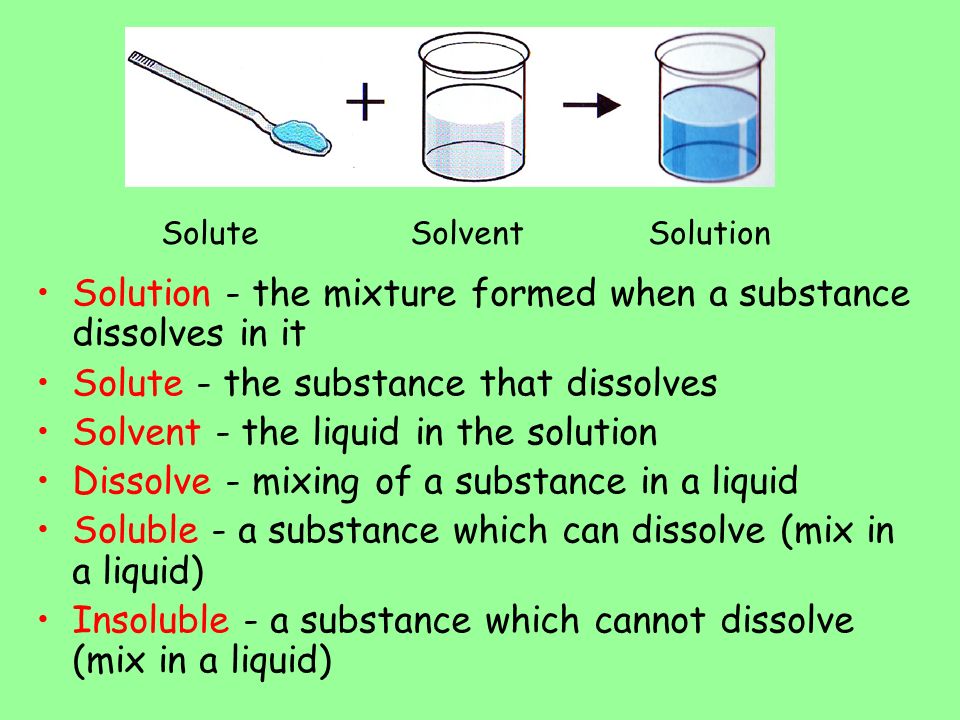
A solute is the substance to be dissolved. A solution is composed of a solute being dissolved in a solvent.
Solutions Solvents Solutes Ppt Video Online Download
A mixture that has more dissolved solute than is predicted by its solubility at the given temp.

. A solvent is a substance that dissolves the solute. Using the illustration below describe the relationship between solute solvent and solution. The solute is the substance that is being dissolved while the solvent is the dissolving medium.
9 rows Describe the relationships between volume and amount of solute to concentration. Hypertonic- a solution where the solute concentration is higher outside the cell. The solute is the substance being dissolved the solvent is the substance dissolve in the solute in the solution is what substance ends up being once it dissolves into the other.
Use molarity to calculate the dilution of solutions. AH STEP 2 exponding the solute solution solute crystol AH. Define the terms solute solvent and solution.
In your definition describe a solution found in your everyday life. When one substance dissolves into another a solution is formedà A solution is a homogeneous mixture of a solute dissolved in a solventà The solute is the substance that dissolves while the solvent is the dissolving mediumà Solutions can be formed with different types of. For example if I pour protein powder the solute.
We know of many types of solutions. Solute A solute is the component of the mixture that is dissolved such as the powdered drink. A material in a solution which is dissolved in a solvent.
In a solution thee substance that gets dissolved in the solvent is called the solute. A homogeneous mixture composed of two or more substances in which a mixture a solute is a substance dissolved in another substance known as a solvent. For example Sugar Water Sugar Solution.
One of them is called the solute. A train robber in a silent western movie steals two gold ingots each of which has a volume of 1000 cm3. A solute is a substance that is dissolved in a solvent.
Calculate the concentration of solutions in units of molarity molL. Sugar Solution Solution. The other is a solvent.
A simple solution is basically two substances that can be combined without a chemical reaction. Its the final product. A solution is a homogeneous mixture containing in it one or more solutes.
Solute and solvent are the two components of a solution. Isotonic - where the solute concentration is in. The solution is created when the particles of the Kool Aid crystals diffuse throughout the water.
Solute Solvent Solution It can be homogeneous with a uniform composition eg an unsaturated solution of a sodium chloride or heterogeneous with non-uniform composition like an unsaturated solution of the same salt. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. The solution of a lpg is gas-solid the solvent mixture of hydrocarbon and the solute is butane.
In your definition describe a solution found in your everyday life. Check out a few examples in the Table below. Explain how solution color and concentration are related.
Compare solubility limits between solutes. Solutions can be formed with many different types and forms of solutes and solvents. CC - Heracles and his labors.
A solvent is a liquid that dissolves a solid liquid or gaseous solute resulting in a solution. Given that the density of gold is 193 gcm3 what is the total mass of the two ingots. It is present in a lesser amount than the solvent.
A mixture that contains as much solute in it as possible at a given temp. Let us define the three terms. Syrup -sugar solute -water solvent.
The solvent is the one doing the dissolution. The water is the solvent and the delicious Kool Aid is the solution. And solutions are a very handy thing to have around.
Solute solvent and solution. Solution is defined as a homogeneous mixture of two or more chemical substances. A simple example to illustrate the definition of solute solvent and solution is a bucket of water containing.
If you make Kool Aid. A solvent is a substance that dissolves a solute in its intermolecular spaces. Generally Solute is in less amount and solvent is in more amount.
Hope This is Helps. What Is The Solute And Solvent Of LPG. Solvent and Solute make a Solution Solvent Solute The Solvent dissolves the Solute substance.
Solvent is basically the thing or substance to which we add our solute to make it a solution. And the solute is the substance being dissolved. A substance which is in liquid form that is dissolved to form a solution.
Expanding the solvent AH STEP 3. Once the solute dissolves into the solvent you have a solution. Solvent- Water Solute - Sugar Solution - Sugar water Solvent - Milk hot Solute - Cocoa powder.
Solution formation liquid solvent. Hypotonic - a solution where the solute concentration is lower when compared to the cell. ScienceFusion Student Edition Interactive Worktext Grades 6-8 Module H.
Solute Solvent Solution Solute S. The powder of Kool Aid crystals are the solute. What Are Examples Of Solvent And Solute.
A solution is the combination of the solute aka particles or stuff and the solvent aka liquid. A liquid mixture which is distributed among the main components. In your definition describe a solution found in your everyday life.
Solution Examples It would probably be easier to tell you what ISNT a solution. The concentration of a solute in a solution is a measure of how much of that solute is dissolved in the solvent with regard to how much solvent is present like salt. Describe what the solvent and solute of a solution are.
View the full answer. Solubility Curve Lab Sheet Pre Lab 1 Define the terms solute solvent and solution. The substance that dissolves the solute in it o a solution is called the solvent.

Year 7 Science Lesson Solute Solvent Solution Edplace Youtube

Solute And Solvent Ck 12 Foundation

Solute Vs Solvent Definition Difference Between Solute And Solvent With Examples
0 Comments